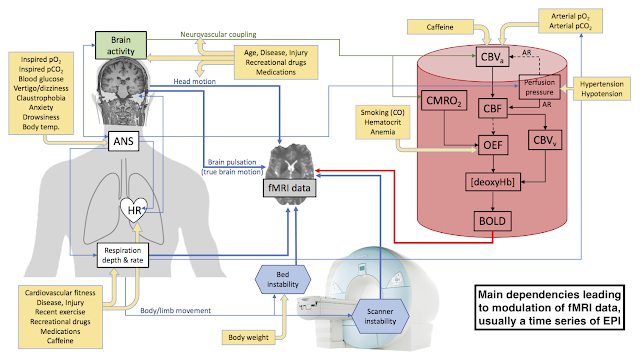
We talk a lot about head motion in fMRI. As much as head motion can be limiting, it’s also important to remember that there is real brain motion, too, distinct from whatever the head might be doing. And whereas printed head cases or bite bars might reduce head motion to a large extent, the real brain motion occurs inside the skull in a way that is inaccessible to anything we can do during data collection. (In principle, one could gate acquisitions to the cardiac cycle, but this would invoke its own set of complications.) Instead, we are forced to deal with real brain motion as best we can during post-processing. The problem here is that our image processing tools usually work at the voxel level, leaving any sub-voxel motion unaddressed.
A few years ago I was involved with a project to assess vibrations measurable on the skull. To relate what could be measured outside with what was happening inside the head, we used a single slice EPI sequence collected at a TR of 40 ms; a frame rate of 25 images per second. At this speed, all the dynamics produced by the arterial blood pressure wave - the mechanical force which propels the blood from the aorta - are visible in quite spectacular fashion right across the brain. Here are a couple of example scans:
Apart from the very obvious fluctuations in the lateral ventricles, note
how the CSF in sulci also fluctuates with each heart beat. If you look
very closely you’ll be able to see the brain tissue deforming, too.
Several vessels, mostly veins, are visible. Pulsation in the
superior sagittal sinus is especially prominent in the sagittal scan. In the transverse scan there are also large changes in the overall image intensity every few heart beats, and this is almost certainly due to breathing (which we didn't track).
Note
that this type of contrast isn’t replicated precisely in your fMRI
scans. Use of a single slice with a relatively high excitation flip
angle (30 degrees) relative to the short TR (40 ms) means that we have
considerable within slice and inflow T1 weighting, in addition to the
T2* changes you’re used to thinking about for BOLD. But some scaled down
version of these apparent T1 changes are in your fMRI data, especially
if you’re not in the habit of using a small flip angle. (See this post and Gonzales-Castillo et al. (2013) for more information on setting the flip angle to minimize motion
effects via image contrast.)
Perhaps more importantly, think about what’s happening
at the tissue level. Even if we somehow magic away all the CSF and large
vessel fluctuations, we’re still left with considerable non-linear
movement of the brain tissue itself. This motion is greatest at the base
of the brain, but displacement and shearing of many cortical areas can
be seen in the above cine loops with the naked eye. Maybe ponder real brain motion the next time you
click the button to apply a motion-correction or physiological noise
reduction step in your processing pipeline. How well do you
think your “motion correction” steps are tackling these low-level
perturbations? Don’t forget they’re also working simultaneously on the
(usually larger) displacements and rotations from real head motion and
pseudo-motion produced by respiration (i.e. chest movements perturbing
the magnetic field across the head).
PS
If you want to see more dynamic images of brain motion, check out the
motion-amplified scans developed by Samantha Holdsworth's group: 2D, 3D, quantitative 3D. Not fMRI but still powerful reminders that the
entire brain is moving almost all the time.